Ivermectin was approved for animal use in 1981, and a few years later it was approved for humans for treatment of roundworm and parasites. Drs. William Campbell and Satoshi Omura received the 2015 Nobel Prize in Physiology and Medicine for development of this drug.
It is now known that besides its antiparasitic and antiviral activity, it also causes immunomodulation, thus making it a candidate drug for treatment of cancers. It is hoped that this immunomodulation effect can also prevent the lethal cytokine storm of severe Covid-19.
The author discusses other studies that have shown that Ivermectin is tolerated at much higher doses than officially approved. 200-400 mcg/kg doses for Dengue fever were safe. A single dose of 120 mg (ten times the appproved dose) and 60 mg given every three days were apparently well tolerated.
Standard Care: In this study,”standard care” included Azithromycin 500 mg for 5 days, 500 mg Paracetamol (Tylenol) as needed, 1,000 mg Vitamin C, 50 mg Zinc, 100 m Lactoferrin twice a day, 200 mg Acetylcystein (used for lung congestion) three times a day. Anticoagulation was used if the D-dimer test was over 1,000.
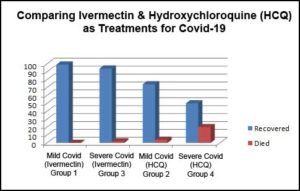
600 people were divided into the following groups:
Group 1 — 100 patients with mild/moderate Covid-19 got 4 days of 400 mcg/kg Ivermectin before breakfast plus standard care 99% recovery; 0% mortality
Group 2 — 100 patients with mild/moderate Covid-19 got 400 mg HCQ every 12 hours the first day followed by 200 mg HCQ every 12 hours for 5 days, plus standard care 74% recovery, 4% mortality
Group 3 — 100 patients with severe Covid-19 got Ivermectin plus standard care (the same treatment as Group 1) 94% recovery, 2% mortality
Group 4 — 100 patients with severe Covid-19 got HCQ plus standard care (the same treatment as Group 2, but for 9 days) 50% recovery, 20% mortality
NOTE: Although an effective early treatment (before hospitalization), HCQ is not nearly as effective once the patient is severely sick.
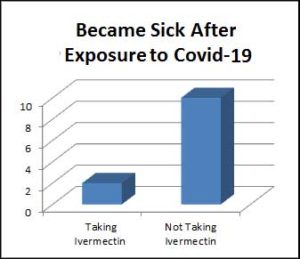 Group 5 –100 household or healthcare people who had been exposed to Covid received PPE (personal protective equipment such as masks) plus a 400 mcg dose of Ivermectin, repeated after 1 week 2% developed Covid-19 infection
Group 5 –100 household or healthcare people who had been exposed to Covid received PPE (personal protective equipment such as masks) plus a 400 mcg dose of Ivermectin, repeated after 1 week 2% developed Covid-19 infection
Group 6 — 100 household or healthcare people who had been exposed to Covis received PPE only 10% developed Covid-19 infection
In Groups 5 and 6, only 2% of exposed household or caretaker contacts taking Ivermectin actually developed Covid-19, while 10% of those not taking Ivermectin got sick.
NOTE: 90% of exposed household/caretaker contacts didn’t get sick, even without any help.
 Research Square says that they “withdrew this preprint on 14 July, 2021 due to an expression of concern communicated directly to our staff. These concerns are now under formal investigation.”
Research Square says that they “withdrew this preprint on 14 July, 2021 due to an expression of concern communicated directly to our staff. These concerns are now under formal investigation.”
The normal procedure would be to contact the authors to ask them to address the “concern” and then publish the “concern” along with the author’s answer to it. To just yank the paper off the Journal without any indication of who expressed this concern, nor what it was, is not the usual scientific approach.