 Journal of Antimicrobial Chemotherapy, 2021 Aug 12;76(9):2415-2418
Journal of Antimicrobial Chemotherapy, 2021 Aug 12;76(9):2415-2418
Several drug treatment trials were reviewed in this paper, including the use of Hydroxychloroquine + Azithromycin, Hydroxychloroquine alone, IL-6 Inhibitors, Corticosteroids, Convalescent Plasma, Remdesivir, and “others” including five antiretroviral drugs and two anti-influenza drugs.
NOTE: Zinc use with the Hydroxychloroquine is not mentioned. Without zinc, the drug cannot work properly.
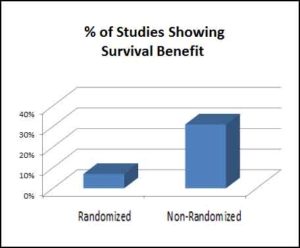
The authors conclude that randomized studies do not replicate the results of non-randomized studies (in which doctors simply treated patients as they appeared). They recommend that healthcare professionals should “resist the pressure to approve and prescribe drugs of unproven efficacy and potential toxicity to optimize patient care and maintain public trust in the quality of medical science.”
Conflicts of Interest: Several of the authors received personal fees, consulting fees, or honorariuims from drug companies including Roche, Pfizer, Novartis, and Oncatest.




















